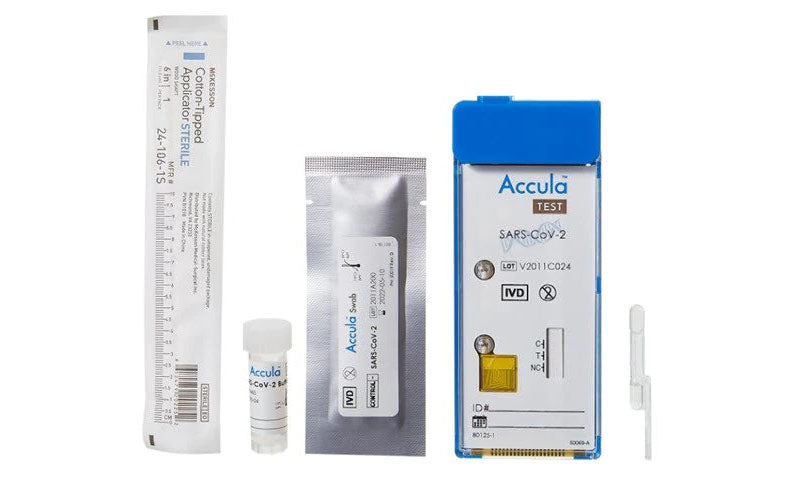
Accula COVID-19 Rapid PCR Test
Description
Currently Out Of Stock
PLEASE NOTE: Production of Accula products have been discontinued by Thermo Fisher.
The Accula™ SARS-CoV-2 Rapid PCR Test is used for the identification of nucleic acids (RNA) of SARS-CoV-2 in human respiratory samples, and will detect SARS-CoV-2 in the presence of human ribonucleic acid (RNA). The test detects viral RNA in a variety of clinical samples and has been shown to yield high sensitivity and specificity in patients with confirmed SARS-CoV-2 infections. Provides results in as little as 30 minutes.
Perfect for international travel or other testing which requires PCR tests!! Pair with our SiteLabs Platform for digital reporting.
**Requires Accula Dock Portable RT-PCR Platform D2000
Must provide a CLIA # to order.
For Point of Care (POC) use in facilities with a CLIA Certificate of Waiver.
FDA EUA Authorized. Not FDA Approved.
**See additional information and disclaimers in the links below.
Key Disclaimer
This test has not been FDA cleared or approved; the test has been authorized by FDA under an Emergency Use Authorization (EUA) for use by laboratories certified under the CLIA that meet the requirements to perform moderate, high or waived complexity tests. This test is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation. A valid CLIA number is required upon checkout.
Refund Policy
The standard refund policy does not apply for COVID-19 rapid tests. All sales are final once goods are shipped and in transit with courier. NO EXCHANGES OR REFUNDS WILL BE MADE.
Product Attachments:
Instructions for Use
Quick Reference Guide
Quick Tips
Point of Care Self-Collection Quick Reference Guide
Dock Operators Guide
Healthcare Provider Fact Sheet
Patient Fact Sheet
FDA EUA Letter of Authorization
Please find additional support and documents on ThermoFisher’s Website.
CPT Code: 87635
Additional information
| Weight | 3 lb |
|---|