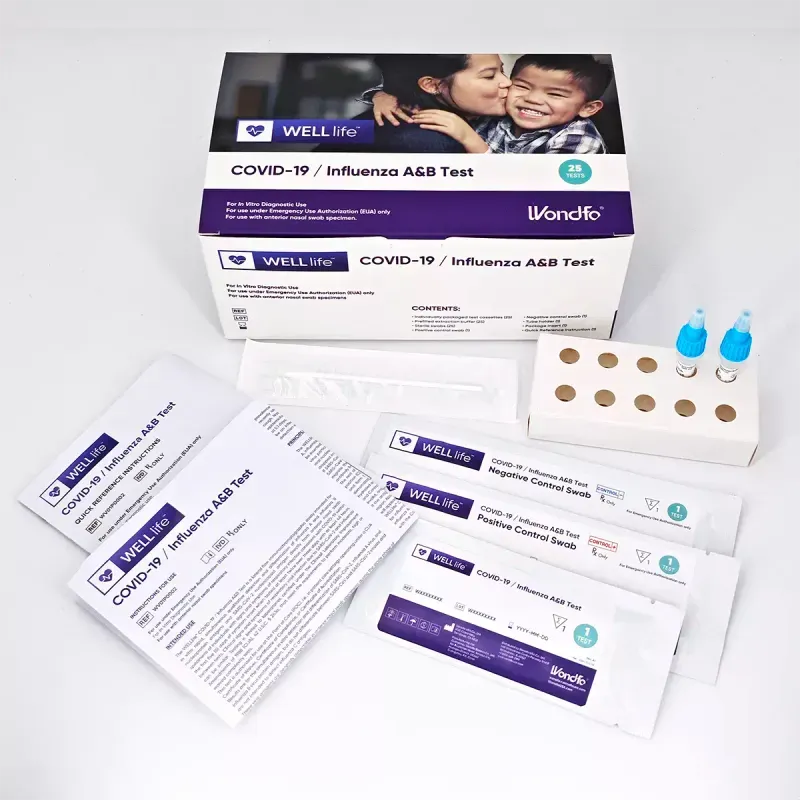
WELLlife™ COVID-19 / Influenza A&B Test Kit (POC)
Rapid test to differentiate between COVID-19, Influenza A, and Influenza B. FDA EUA-authorized and CLIA-waived for Point-of-Care (POC) use.
Description
The WELLlife™ COVID-19 / Influenza A&B Test differentiates between SARS-COV-2, influenza A and influenza B antigens with a single test.
Each kit includes 25 tests and 1 positive and 1 negative control swab test.
This product is for professional/POC use only.
Features and Benefits
Cost-effective
3-in-1 test differentiate between SARS-COV-2, influenza A, and influenza B antigens with a single test using an anterior nasal swab specimen.
Rapid
Results available in 10 minutes allow for testing and treatment decision-making during the same office visit.
Quality Assurance
The outstanding clinical performance, including built-in kit controls for external quality testing, enables physicians to make quicker and more confident decisions.
Extended Detection Window
Offers a broader detection window for differentiation of SARS-COV-2, influenza A, and influenza B antigens within five (5) days of symptom onset
Wide Storage Temperature
36°F-86°F(2°C-30°C) allows for easier product storage.
Performance Characteristics

Easy to Use

Rapid results in just 10 minutes!
Clear Interpretation

HCPCS Code: 87428
Average Reimbursement Amount: $70.29
Emergency Use Authorization (EUA240004)
You must be logged in to post a review.

Reviews
There are no reviews yet.